Calcium homeostasis workgroup
Group leader: Prof. Dr. László Csernoch
Post docs: Beatrix Dienes, Nóra Dobrosi, János Fodor, Mónika Gönczi, Péter Szentesi, Mónika Sztretye, Andrea Telek
Ph.D. students: Ágnes Angyal, Bernadett
Bákány, Norbert Balogh, Zsolt Ráduly, Zoltán
Singlár, László Szabó
Technicians: Tamara Lővei, Róza Őri, Anita Szabóné Jeney
Selected ongoing projects
Selenium supplementation improves
skeletal muscle performance
As an essential trace element selenium plays an important role in many physiological functions of
the organs. It is found within muscles as selenocystein
in selenoprotein N, which is involved in redox-modulated calcium homeostasis
and in protection against oxidative stress. We tested the effects of two
different selenium compounds (selenate
and NanoSe) on muscle properties of
mice. Selenium diets significantly increased the speed of voluntary running and
the daily distance covered. Both forms of selenium increased significantly the
amplitude of single twitches in EDL and SOL muscle. The amplitude of the
calcium transients evoked by KCl depolarization increased significantly in the
presence of selenate in FDB fibers. In parallel, the rate of calcium release
during short depolarizations increased significantly in the presence of NanoSe
and selenate. Both selenium compounds increased significantly the selenoprotein
N expression in EDL muscle (Figure 1).
Thus we concluded, that selenium supplementation augments calcium release from
the sarcoplasmic reticulum and improves skeletal muscle performance. The
increased selenoprotein N expression in the muscles could result in increased
oxidative stress tolerance in case of long lasting contraction.
|
|
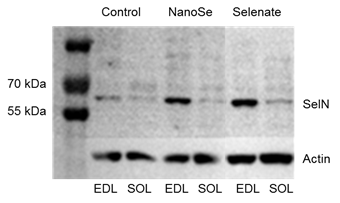
Figure 1. Representative
Western blot image showing the expression of selenoprotein N (SelN) in EDL and SOL muscles from a control mouse and a
mouse fed with 0.5 ppm NanoSe or selenate for two weeks |
Hypermuscular mice
display impared EC coupling
Myostatin, a member of the transforming growth factor β
superfamily has emerged as a potent negative regulator of skeletal muscle
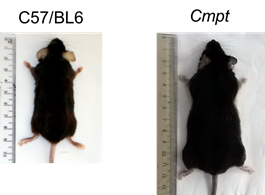
growth. It is strongly expressed in skeletal muscle and Cmpt mice have a great increase in muscle mass (Figure 2) demonstrating that myostatin
is a muscle-specific negative regulator of skeletal muscle size and it also
regulates muscle mass in adult mice.
|
|
Figure 2. A control C57/BL6
(left) and a Cmpt (right) mouse. |
Cmpt
mice display excessive muscle mass and this is associated with a profound loss
of oxidative metabolic properties. In grip tests the myostatin-deficient mice
showed higher average absolute force than the control mice. In voluntary wheel
running the control mice performed better showing higher average and maximal
speed, and total running distance. The amplitude of single twitches in EDL and
SOL is higher in the control strain. The Ca2+-sensitivity of force
production was not significantly difference between the two mouse strains.
While resting intracellular Ca2+ concentration measured on single intact flexor
digitorum brevis (FDB) muscle fibres was identical to
control, the amplitude of depolarization-evoked calcium transients was smaller
in the mutant strain (Figure 3). SR
calcium release flux, calculated from these transients showed a reduced peak
and steady level with no change in the peak-to-steady ratio. The amplitude and
spatial spread of spontaneous elementary calcium release events detected on
permeabilized FDB fibres were also significantly
smaller in mutant mice. These results indicate that alterations in calcium
signaling may underlie the reduced muscle performance in Cmpt animals.
|
|
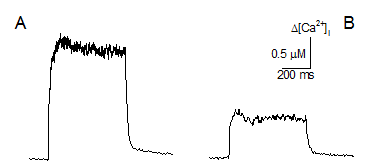
Figure 3. Calcium transients
were elicited by a supra-threshold pulse in intact FDB fibres from a control (A) and Cmpt (B) mouse. |
SOCE is important during normal EC
coupling
We are interested in the characterization of store operated calcium entry (SOCE) in
the Cmpt mouse model. Our aim is to
understand the role of SOCE in refilling SR Ca2+ stores in skeletal
muscle which is currently fairly controversial. We recently demonstrated that
the naturally occurring Cmpt mutation
leads to reduced Ca2+ store content (Figure 4) that could be responsible for the reduced specific force.
The most likely explanation for how the mutation leads to this reduction is the
reduced expression of the SOCE partner proteins and the concomitant lower SOCE
activity. We propose that SOCE has a role in maintaining and refilling SR Ca2+
stores not only in repetitive tetanic stimulation, as it was previously
reported, but on an immediate basis in agreement with latest observations.
|
|
|
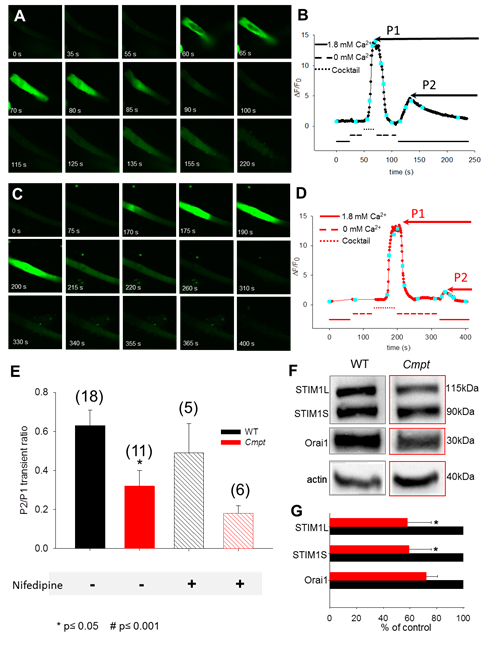
Figure
4. Representative xy images
showing the response to various solution exchanges and the consequent SOCE
activation in enzymatically isolated FDB fibers of C57/BL6 wild type (A)
and Cmpt (C) mouse. The
averaged profile of fluo-8 fluorescence over the fibers was used to assess
the cytoplasmic [Ca2+]. Intracellular Ca2+ stores were
emptied using a depleting cocktail in a Ca2+-free medium resulting
in a massive Ca2+ -release from the SR via RyRs. (B, D)
During manual solution exchanges the fluorescence profile vs. elapsed
time was plotted. Each confocal image from panel A and C is depicted by the
cyan squares. When returning to the 1.8 mM Ca2+ in the external
solution, a secondary increase in fluorescence was detected, indicating
perhaps SOCE activation and Ca2+-influx via activated Orai1
channels in the sarcolemma. (E) The ratio distribution
between the peak of the slow Ca2+ transient highlighting the
activation of SOCE (P2) and the depleting cocktail induced Ca2+ transient´s
peak (P1). 1 µM nifedipine (L-type Ca2+ channel
blocker) the Ca2+ influx was abolished in the mutant suggesting
some contribution via the DHPR, a process also known as excitation-coupled
calcium entry (ECCE). The number of cells examined is given in parenthesis.
For statistical analysis, the paired T-test was used, and p ≤0.05 was considered significant. (F) Representative Western blot illustrating the relative
expression levels of STIM1 and Orai1, identified as the key proteins involved
in SOCE. Note: the presence of two distinct STIM1 proteins: the
well-known 90 kDa STIM1S (S denoting small) and the newly identified, widely
expressed 115 kDa STIM1L isoform (L for long). 20 µg of whole FDB
muscle homogenate was loaded into each lane and immunoreactivity with actin
was used as an internal control. (G) In 4 independent experiments the
STIM1 and Orai1 endogenous proteins level distribution was assessed as a
percentage of control. On average, both STIM1 and Orai1 were decreased by 40
% and 27% respectively, in the Cmpt muscles. |
Selected references:
Bodnár
D., Geyer N., Ruzsnavszky O., Oláh
T., Hegyi B., Sztretye M.,
Fodor J., Dienes B., Balogh Á., Papp Z., Szabó L., Müller G., Csernoch L., Szentesi P. (2014) Hypermuscular mice with mutation in the myostatin gene
display altered calcium signaling. Journal of Physiology, 592: 1353-1365. doi: 10.1113/jphysiol.2013.261958.
Bodnár
D., Ruzsnavszky O., Oláh
T., Dienes B., Balatoni I., Ungvári
É., Benkő I.., Babka B., Prokisch J., Csernoch L., Szentesi P. (2016) Dietary
selenium augments sarcoplasmic calcium release and mechanical performance in
mice. Nutrition and Metabolism, 13:76. doi:
10.1186/s12986-016-0134-6
Sultana N., Dienes B., Benedetti A., Tuluc P., Szentesi P., Sztretye
M., Rainer J., Hess M.W., Schwarzer C., Obermair G.J., Csernoch L., Flucher
B.E. (2016) Restricting calcium currents is required for correct fiber type
specification in skeletal muscle. Development, 143: 1547-1559. doi: 10.1242/dev.129676.
Sztretye M., Geyer N., Vincze J.,
Al-Gaadi D., Oláh T.,
Szentesi P., Kis G., Balatoni
I., Csernoch L., Dienes B. (2017) Store-operated calcium entry is important for
maintaining sarcoplasmic calcium content and release in mammalian skeletal
muscle fibers. Biophysical Journal, 113:2496-2507. doi: 10.1016/j.bpj.2017.09.023.
Pierantozzi E., Szentesi P., Al-Gaadi
D., Oláh T., Dienes B., Sztretye
M., Rossi D., Sorrentino V., Csernoch L. (2019)
Calcium homeostasis is modified in skeletal muscle fibers of small Ankyrin1
knockout mice. International Journal of Molecular Sciences, 20(13):pii: E3361. doi: 10.3390/ijms20133361.
Fodor J., Al-Gaadi D., Czirják T., Oláh T., Dienes B.,
Csernoch L., Szentesi P. (2020) Improved calcium homeostasis and force by
selenium treatment and training in aged mouse skeletal muscle. Scientific
Reports, 10(1):1707. doi:
10.1038/s41598-020-58500-x.
Collaborations:
Claude Collet, National Institute for Agricultural
Research, Avignon, France
Laszlo Dux, University of Szeged, Hungary
Bernhard Flucher, University
of Innsbruck, Austria
Vincent Jacquemond,
University of Lyon, France
Vincenzo Sorrentino, University of Siena, Italy
Available techniques:
Intracellular ion concentration measurement with
fluorescence dyes on confocal microscope
Electrophysiological measurement on isolated single
skeletal muscle fibers
In vivo muscle grip force, voluntary and forced treadmill
running on mice
In vitro muscle force measurement on isolated skeletal muscle
Immunohistochemistry measurement on tissues and cells
Molecular biology techniques